Tutorial: Registering your IRB protocol with the HIRO (if you are the Principal Investigator)
Registering an IRB-approved research protocol with the HIRO is necessary if you wish to utilize any of the HIRO's services. If you are the Principal Investigator (PI) or a Co-Investigator of a research protocol and you would like to register it with the HIRO, please follow the simple steps below.
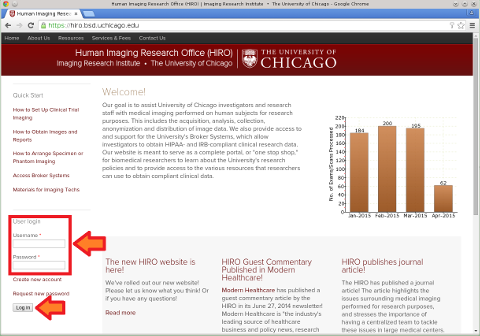
(1) Log in to the HIRO website using your CNet ID
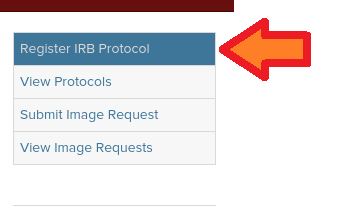
(2) Click the Register IRB Protocol button
Click the Register IRB Protocol button on the left side of the website.
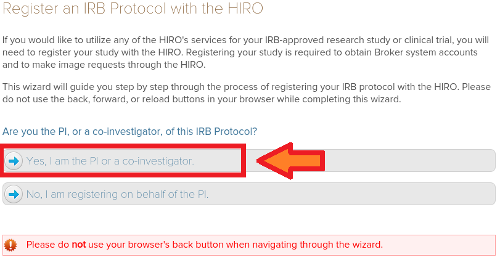
(3) Click the Yes, I am the PI or a Co-PI option
(4) Complete the registration process
Complete the registration process by answering the questions that follow. Once you have finished answering these questions, you will be given the option to review and submit your registration request.
(5) Add personnel to the protocol
After you submit your request, you will be given the option of adding personnel to the study and/or assigning access to HIRO services. You can perform these steps at any time, but you will not be able to request any actual services (like copies of images) until your registration request has been approved.
(6) Await protocol verification
Once your request has been submitted, it will be added to the HIRO's review queue. The details of your request will be verified via the AURA IRB system and the Office of Clinical Research (OCR). This is usually completed within 2 business days. Please note that the HIRO will not review the scientific validity of the protocol nor will they verify if the protocol permits the use of image data; they will only verify the demographic details of the protocol and its approval status.
(7) Protocol approved!
You will be informed via email when your registration request has been approved. Once it is approved, you can start requesting services like image requests for the protocol.
If you would like to register a research protocol via paper form:
When required, the HIRO offers an alternative, paper-based protocol registration method. This method is not preferred and is generally discouraged, but it is available when necessary.
- You should complete a HIRO Research Protocol Registration Form for the study. The completed form should be returned to the HIRO.
- The HIRO will set up the appropriate research protocol registration in its web system and verify the protocol's details as above.
- If you do not already have a HIRO account, a dummy account can be created for you by HIRO staff during the protocol registration process. This is not ideal; you are encouraged to create a HIRO account whenever possible.
The HIRO will contact all parties involved when the process is complete. You will be able to request services against the protocol, and you will have ultimate administrative control over it in the HIRO's web system (i.e., you can add and remove study personnel and assign access to services).
